As someone in the polyurea business, I have grown used to having to explain what Polyurea is in pretty simple terms so that people have a basic understanding of what it is. I decided it would be helpful to give you both a simplified and a more comprehensive explanation for what Polyurea is.
Polyurea provides the best characteristics of many different compounds. At twenty times stronger than most epoxy coatings, it is extremely durable.
It’s chemically resistant to salt, oil, fuel and many other harsh chemicals. It is also 98% more flexible than epoxy, which allows for extreme movement however it won’t crack or peel. If all that didn’t seal the deal, Polyurea also installs in less than half the time and are environmentally ‘green’ compared to competing epoxy coatings.
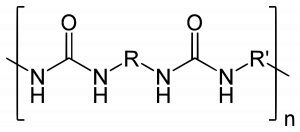
Polyurea is an organic polymer that is the reaction of isocyanate with an amine terminated polyether resin, forming a plastic-like or rubber-like compound that may be used in many of the same ways as older technologies – polyurethane, epoxy, vinyl ester, neoprene; to name a few.
What is Polyurea? – As defined by the PDA
A polyurea coating/elastomer is that derived from the reaction product of an isocyanate component and a resin blend component. The isocyanate can be aromatic or aliphatic in nature. It can be monomer, polymer, or any variant reaction of isocyanates, quasi-prepolymer or a prepolymore. The prepolymer, or quasi-prepolymer, can be made of an amine-terminated polymer resin, or a hydroxyl-terminated polymer resin.
The Resin blend must be made up of amine-terminated polymer resins, and/or amineterminated chain extenders. The amine-terminated polymer resins will not have any intentional hydroxyl moieties. Any hydroxyls are the result of incomplete conversion to the amine-terminated polymer resins. The resin blend may also contain additives, or non-primary components. These additives may contain hydroxyls, such as pre-dispersed pigments in a polyol carrier. Normally, the resin blend will not contain a catalyst(s).